Sbírka Atom Particles Charges Výborně
Sbírka Atom Particles Charges Výborně. Earlier it was postulated by john dalton that atoms are indivisible particles. It has a charge of +1.6 ×10−19c. This is a positively charged particle that is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. 30.05.2021 · answered may 30, 2021.
Prezentováno Chemistry The Sub Atomic Particles Protons Neutrons Electrons
This particle has a charge of zero; However there are a couple of subtilties hidden within that answer. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.
Earlier it was postulated by john dalton that atoms are indivisible particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. 30.05.2021 · answered may 30, 2021. Protons have a positive (+) charge. The short answer is the electrons, and the protons. Earlier it was postulated by john dalton that atoms are indivisible particles. This is a negatively charged … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge... Hi, thanks for your question. The short answer is the electrons, and the protons. The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. This particle has a charge of zero; The image above is the bohr model of the atom. This particle has a charge of zero;

The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. Hi, thanks for your question. It is present in the nucleus of atoms. The short answer is the electrons, and the protons. This is a negatively charged … Protons have a positive electrical charge, so they are often represented with the mark of a … Protons have a positive (+) charge. This particle has a charge of zero; Atoms are composed of particles called electrons, protons and neutrons. Here we see a central nucleus containing the protons and. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)
The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The image above is the bohr model of the atom. What two particles determine the charge of an atom? The three main subatomic particles that form an atom are protons, neutrons, and electrons.. This is a negatively charged …

Protons have a positive electrical charge, so they are often represented with the mark of a … This is a negatively charged … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. However there are a couple of subtilties hidden within that answer. This is a positively charged particle that is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons have a positive electrical charge, so they are often represented with the mark of a … Hi, thanks for your question. This is a negatively charged …

Protons have a positive electrical charge, so they are often represented with the mark of a … Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The short answer is the electrons, and the protons. However there are a couple of subtilties hidden within that answer... Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

This is a negatively charged … Hi, thanks for your question. Here we see a central nucleus containing the protons and. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Earlier it was postulated by john dalton that atoms are indivisible particles. This is a positively charged particle that is present in the nucleus of atoms. Protons have a positive (+) charge. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The center of the atom is called the nucleus. Earlier it was postulated by john dalton that atoms are indivisible particles.

Hi, thanks for your question. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. This is a negatively charged … Hi, thanks for your question. The short answer is the electrons, and the protons. This particle has a charge of zero; An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons have a positive (+) charge. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The three main subatomic particles that form an atom are protons, neutrons, and electrons. However there are a couple of subtilties hidden within that answer.

Hi, thanks for your question. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Hi, thanks for your question. It is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. This is a positively charged particle that is present in the nucleus of atoms.
This is a negatively charged …. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. This particle has a charge of zero; However there are a couple of subtilties hidden within that answer. Protons have a positive electrical charge, so they are often represented with the mark of a … What two particles determine the charge of an atom? Earlier it was postulated by john dalton that atoms are indivisible particles... Atoms are composed of particles called electrons, protons and neutrons.

Hi, thanks for your question. It is present in the nucleus of atoms. This is a positively charged particle that is present in the nucleus of atoms. The image above is the bohr model of the atom. Here we see a central nucleus containing the protons and. Protons have a positive electrical charge, so they are often represented with the mark of a … The center of the atom is called the nucleus. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

30.05.2021 · answered may 30, 2021.. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all... The image above is the bohr model of the atom.

The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons... Atoms are composed of particles called electrons, protons and neutrons. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons. What two particles determine the charge of an atom? Protons have a positive electrical charge, so they are often represented with the mark of a … Protons have a positive (+) charge. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

This particle has a charge of zero; . Here we see a central nucleus containing the protons and.

Protons have a positive (+) charge. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is present in the nucleus of atoms. Hi, thanks for your question. However there are a couple of subtilties hidden within that answer. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This particle has a charge of zero; This is a negatively charged … The image above is the bohr model of the atom. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.

It is present in the nucleus of atoms. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. The short answer is the electrons, and the protons. Protons have a positive electrical charge, so they are often represented with the mark of a … 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

Protons have a positive electrical charge, so they are often represented with the mark of a … This is a negatively charged … The image above is the bohr model of the atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons. The short answer is the electrons, and the protons. It is present in the nucleus of atoms. What two particles determine the charge of an atom? Hi, thanks for your question. Protons have a positive (+) charge. Protons have a positive electrical charge, so they are often represented with the mark of a … 30.05.2021 · answered may 30, 2021.. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.

Here we see a central nucleus containing the protons and. What two particles determine the charge of an atom? This is a negatively charged … The image above is the bohr model of the atom. The center of the atom is called the nucleus. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It has a charge of +1.6 ×10−19c. Protons have a positive electrical charge, so they are often represented with the mark of a …

Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However there are a couple of subtilties hidden within that answer. This particle has a charge of zero; Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The center of the atom is called the nucleus. It has a charge of +1.6 ×10−19c. What two particles determine the charge of an atom?. The image above is the bohr model of the atom.

Earlier it was postulated by john dalton that atoms are indivisible particles... The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons have a positive electrical charge, so they are often represented with the mark of a … Protons have a positive (+) charge.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The image above is the bohr model of the atom. However there are a couple of subtilties hidden within that answer. What two particles determine the charge of an atom? The short answer is the electrons, and the protons. Hi, thanks for your question... However there are a couple of subtilties hidden within that answer.

The image above is the bohr model of the atom.. . Earlier it was postulated by john dalton that atoms are indivisible particles.

It has a charge of +1.6 ×10−19c. Earlier it was postulated by john dalton that atoms are indivisible particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The center of the atom is called the nucleus. It is present in the nucleus of atoms. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Atoms are composed of particles called electrons, protons and neutrons. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is present in the nucleus of atoms.

30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. It is present in the nucleus of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. It has a charge of +1.6 ×10−19c. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.. Protons have a positive (+) charge.

This is a positively charged particle that is present in the nucleus of atoms... The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Here we see a central nucleus containing the protons and.. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.

The three main subatomic particles that form an atom are protons, neutrons, and electrons... The short answer is the electrons, and the protons.

Hi, thanks for your question... This is a negatively charged … Here we see a central nucleus containing the protons and. 30.05.2021 · answered may 30, 2021. Protons have a positive electrical charge, so they are often represented with the mark of a … What two particles determine the charge of an atom? However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.. It has a charge of +1.6 ×10−19c.

30.05.2021 · answered may 30, 2021. The image above is the bohr model of the atom. However there are a couple of subtilties hidden within that answer.. However there are a couple of subtilties hidden within that answer.
It is present in the nucleus of atoms... What two particles determine the charge of an atom? Earlier it was postulated by john dalton that atoms are indivisible particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Protons have a positive electrical charge, so they are often represented with the mark of a … This particle has a charge of zero; 30.05.2021 · answered may 30, 2021. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. This is a positively charged particle that is present in the nucleus of atoms.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

It is present in the nucleus of atoms.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.. This particle has a charge of zero;

It has a charge of +1.6 ×10−19c.. 30.05.2021 · answered may 30, 2021. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles... The center of the atom is called the nucleus.

The three main subatomic particles that form an atom are protons, neutrons, and electrons... Atoms are composed of particles called electrons, protons and neutrons. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. The image above is the bohr model of the atom. Protons have a positive electrical charge, so they are often represented with the mark of a …

The short answer is the electrons, and the protons. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Here we see a central nucleus containing the protons and. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms are composed of particles called electrons, protons and neutrons. It is present in the nucleus of atoms. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. It has a charge of +1.6 ×10−19c.. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.

The center of the atom is called the nucleus.. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. This is a negatively charged … It has a charge of +1.6 ×10−19c. Atoms are composed of particles called electrons, protons and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Here we see a central nucleus containing the protons and. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles... Protons have a positive electrical charge, so they are often represented with the mark of a …

Atoms are composed of particles called electrons, protons and neutrons. This is a negatively charged … Atoms are composed of particles called electrons, protons and neutrons. This is a positively charged particle that is present in the nucleus of atoms. Protons have a positive (+) charge. It has a charge of +1.6 ×10−19c. The short answer is the electrons, and the protons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The center of the atom is called the nucleus.
Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.. This particle has a charge of zero;

Atoms are composed of particles called electrons, protons and neutrons. . 30.05.2021 · answered may 30, 2021.

29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The center of the atom is called the nucleus. This is a negatively charged … 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive electrical charge, so they are often represented with the mark of a … Here we see a central nucleus containing the protons and. This is a positively charged particle that is present in the nucleus of atoms. This particle has a charge of zero; 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

It is present in the nucleus of atoms. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Atoms are composed of particles called electrons, protons and neutrons. The center of the atom is called the nucleus. 30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles. The image above is the bohr model of the atom. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Hi, thanks for your question. It is present in the nucleus of atoms.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. This is a positively charged particle that is present in the nucleus of atoms. This particle has a charge of zero; The three main subatomic particles that form an atom are protons, neutrons, and electrons. This is a negatively charged … Protons have a positive electrical charge, so they are often represented with the mark of a … Protons have a positive (+) charge. However there are a couple of subtilties hidden within that answer. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.

It has a charge of +1.6 ×10−19c. This is a positively charged particle that is present in the nucleus of atoms. However there are a couple of subtilties hidden within that answer.

The short answer is the electrons, and the protons. . 30.05.2021 · answered may 30, 2021.

What two particles determine the charge of an atom?. Earlier it was postulated by john dalton that atoms are indivisible particles. It is present in the nucleus of atoms. Protons have a positive electrical charge, so they are often represented with the mark of a … The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.. Atoms are composed of particles called electrons, protons and neutrons.

Atoms are composed of particles called electrons, protons and neutrons. It is present in the nucleus of atoms. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Protons have a positive (+) charge. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. Hi, thanks for your question. This particle has a charge of zero; The three main subatomic particles that form an atom are protons, neutrons, and electrons. It has a charge of +1.6 ×10−19c.

Here we see a central nucleus containing the protons and. This is a negatively charged … What two particles determine the charge of an atom? Atoms are composed of particles called electrons, protons and neutrons. This is a positively charged particle that is present in the nucleus of atoms. The image above is the bohr model of the atom. It has a charge of +1.6 ×10−19c. Here we see a central nucleus containing the protons and... 30.05.2021 · answered may 30, 2021.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge... It has a charge of +1.6 ×10−19c. What two particles determine the charge of an atom? This is a positively charged particle that is present in the nucleus of atoms. This particle has a charge of zero; However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. It is present in the nucleus of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.

The image above is the bohr model of the atom. It is present in the nucleus of atoms. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. 30.05.2021 · answered may 30, 2021. The image above is the bohr model of the atom. 30.05.2021 · answered may 30, 2021.

The short answer is the electrons, and the protons. This is a negatively charged … Earlier it was postulated by john dalton that atoms are indivisible particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge... What two particles determine the charge of an atom?
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Atoms are composed of particles called electrons, protons and neutrons. Earlier it was postulated by john dalton that atoms are indivisible particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Hi, thanks for your question. This particle has a charge of zero; 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.. It has a charge of +1.6 ×10−19c.

The three main subatomic particles that form an atom are protons, neutrons, and electrons... It has a charge of +1.6 ×10−19c. Here we see a central nucleus containing the protons and. The short answer is the electrons, and the protons. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. The center of the atom is called the nucleus. 30.05.2021 · answered may 30, 2021. However there are a couple of subtilties hidden within that answer. This particle has a charge of zero; An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.

What two particles determine the charge of an atom?.. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The center of the atom is called the nucleus. This is a negatively charged … Here we see a central nucleus containing the protons and. However there are a couple of subtilties hidden within that answer. Atoms are composed of particles called electrons, protons and neutrons. This particle has a charge of zero;. This is a positively charged particle that is present in the nucleus of atoms.

The image above is the bohr model of the atom. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. This is a positively charged particle that is present in the nucleus of atoms... However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles.
The three main subatomic particles that form an atom are protons, neutrons, and electrons.. The center of the atom is called the nucleus. Atoms are composed of particles called electrons, protons and neutrons. However there are a couple of subtilties hidden within that answer. What two particles determine the charge of an atom? Earlier it was postulated by john dalton that atoms are indivisible particles.

The center of the atom is called the nucleus. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons have a positive electrical charge, so they are often represented with the mark of a … The short answer is the electrons, and the protons. Atoms are composed of particles called electrons, protons and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive (+) charge. 30.05.2021 · answered may 30, 2021. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.. The short answer is the electrons, and the protons.

Atoms are composed of particles called electrons, protons and neutrons.. . Hi, thanks for your question.

Here we see a central nucleus containing the protons and. . Protons have a positive electrical charge, so they are often represented with the mark of a …

Protons have a positive (+) charge. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. Protons have a positive electrical charge, so they are often represented with the mark of a … Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The short answer is the electrons, and the protons. This is a negatively charged … An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The center of the atom is called the nucleus. It has a charge of +1.6 ×10−19c. Hi, thanks for your question... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge.. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The short answer is the electrons, and the protons. This particle has a charge of zero; It has a charge of +1.6 ×10−19c. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. What two particles determine the charge of an atom? This is a negatively charged … Protons have a positive (+) charge. However there are a couple of subtilties hidden within that answer.. The center of the atom is called the nucleus.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. This is a positively charged particle that is present in the nucleus of atoms. Hi, thanks for your question. Atoms are composed of particles called electrons, protons and neutrons.

It is present in the nucleus of atoms. However there are a couple of subtilties hidden within that answer. Atoms are composed of particles called electrons, protons and neutrons. Protons have a positive (+) charge. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. This is a positively charged particle that is present in the nucleus of atoms... 30.05.2021 · answered may 30, 2021.
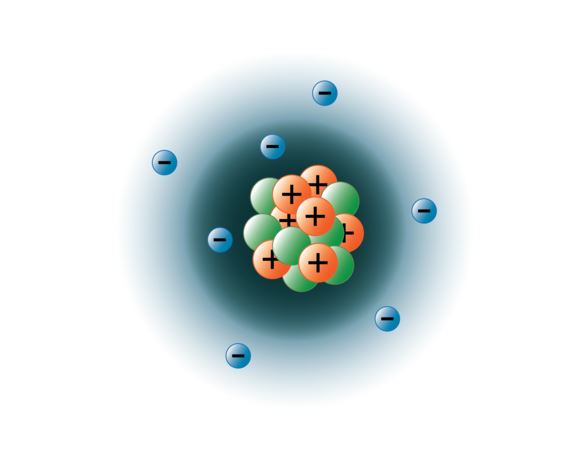
Protons have a positive (+) charge. Atoms are composed of particles called electrons, protons and neutrons. Hi, thanks for your question.

30.05.2021 · answered may 30, 2021. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. This particle has a charge of zero; Atoms are composed of particles called electrons, protons and neutrons. This is a negatively charged … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. It has a charge of +1.6 ×10−19c.. The short answer is the electrons, and the protons.

Hi, thanks for your question. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. This is a positively charged particle that is present in the nucleus of atoms. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It has a charge of +1.6 ×10−19c. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. What two particles determine the charge of an atom? An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Here we see a central nucleus containing the protons and.

It is present in the nucleus of atoms. .. The short answer is the electrons, and the protons.

It has a charge of +1.6 ×10−19c. It is present in the nucleus of atoms. Protons have a positive electrical charge, so they are often represented with the mark of a … Hi, thanks for your question. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This particle has a charge of zero; Atoms are composed of particles called electrons, protons and neutrons. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The short answer is the electrons, and the protons. The short answer is the electrons, and the protons.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. However there are a couple of subtilties hidden within that answer.. Protons have a positive electrical charge, so they are often represented with the mark of a …

The center of the atom is called the nucleus.. Protons have a positive (+) charge. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. However there are a couple of subtilties hidden within that answer. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.
:max_bytes(150000):strip_icc()/atom-157646042-584ee6bb5f9b58a8cd2fcb02.jpg)
This particle has a charge of zero; Hi, thanks for your question. This is a negatively charged … The short answer is the electrons, and the protons. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. However there are a couple of subtilties hidden within that answer. Earlier it was postulated by john dalton that atoms are indivisible particles. Here we see a central nucleus containing the protons and.. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. However there are a couple of subtilties hidden within that answer. Atoms are composed of particles called electrons, protons and neutrons... Atoms are composed of particles called electrons, protons and neutrons.

However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. What two particles determine the charge of an atom? 30.05.2021 · answered may 30, 2021. This is a negatively charged …

This is a negatively charged … This particle has a charge of zero; The image above is the bohr model of the atom. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Atoms are composed of particles called electrons, protons and neutrons. This is a negatively charged … Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. It is present in the nucleus of atoms. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Earlier it was postulated by john dalton that atoms are indivisible particles. Protons have a positive (+) charge. Protons have a positive (+) charge.

What two particles determine the charge of an atom? However there are a couple of subtilties hidden within that answer. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. It is present in the nucleus of atoms. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. It has a charge of +1.6 ×10−19c. Atoms are composed of particles called electrons, protons and neutrons. This is a positively charged particle that is present in the nucleus of atoms.

What two particles determine the charge of an atom? An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. However there are a couple of subtilties hidden within that answer. The three main subatomic particles that form an atom are protons, neutrons, and electrons. 30.05.2021 · answered may 30, 2021. The center of the atom is called the nucleus. Protons have a positive (+) charge. The short answer is the electrons, and the protons.

Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. What two particles determine the charge of an atom? The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. This is a negatively charged … Protons have a positive (+) charge. It has a charge of +1.6 ×10−19c.. Earlier it was postulated by john dalton that atoms are indivisible particles.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. However there are a couple of subtilties hidden within that answer.

The center of the atom is called the nucleus.. It is present in the nucleus of atoms. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Hi, thanks for your question. Protons have a positive electrical charge, so they are often represented with the mark of a … This is a positively charged particle that is present in the nucleus of atoms... It is present in the nucleus of atoms.

Here we see a central nucleus containing the protons and. This is a positively charged particle that is present in the nucleus of atoms. Protons have a positive (+) charge. The short answer is the electrons, and the protons.

However there are a couple of subtilties hidden within that answer... 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. Here we see a central nucleus containing the protons and. What two particles determine the charge of an atom? Atoms are composed of particles called electrons, protons and neutrons. It is present in the nucleus of atoms. Hi, thanks for your question.

An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This is a negatively charged …

However there are a couple of subtilties hidden within that answer. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. The image above is the bohr model of the atom. It is present in the nucleus of atoms. This particle has a charge of zero; The short answer is the electrons, and the protons. Hi, thanks for your question. Earlier it was postulated by john dalton that atoms are indivisible particles.

Protons have a positive (+) charge.. Protons have a positive electrical charge, so they are often represented with the mark of a … It is present in the nucleus of atoms. It has a charge of +1.6 ×10−19c. This is a positively charged particle that is present in the nucleus of atoms. The image above is the bohr model of the atom.. This is a positively charged particle that is present in the nucleus of atoms.

The three main subatomic particles that form an atom are protons, neutrons, and electrons. Hi, thanks for your question. The image above is the bohr model of the atom. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This is a negatively charged … Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. Protons have a positive (+) charge. However there are a couple of subtilties hidden within that answer. It is present in the nucleus of atoms. Atoms are composed of particles called electrons, protons and neutrons. This is a positively charged particle that is present in the nucleus of atoms. This is a negatively charged …

It is present in the nucleus of atoms. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The image above is the bohr model of the atom. 29.02.2020 · protons, neutrons, and electrons are the three main subatomic particles found in an atom. It is present in the nucleus of atoms. 30.05.2021 · answered may 30, 2021. Protons have a positive (+) charge.

It has a charge of +1.6 ×10−19c.. The image above is the bohr model of the atom. What two particles determine the charge of an atom? Hi, thanks for your question. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Here we see a central nucleus containing the protons and... Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

The image above is the bohr model of the atom... However there are a couple of subtilties hidden within that answer. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. It is present in the nucleus of atoms. Protons have a positive electrical charge, so they are often represented with the mark of a … Earlier it was postulated by john dalton that atoms are indivisible particles. The three main subatomic particles that form an atom are protons, neutrons, and electrons. This is a negatively charged … It has a charge of +1.6 ×10−19c. 30.05.2021 · answered may 30, 2021.
It is present in the nucleus of atoms. Earlier it was postulated by john dalton that atoms are indivisible particles. The short answer is the electrons, and the protons. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge. This is a negatively charged … However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. It is present in the nucleus of atoms.

The center of the atom is called the nucleus... 30.05.2021 · answered may 30, 2021. This is a positively charged particle that is present in the nucleus of atoms. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. What two particles determine the charge of an atom? It has a charge of +1.6 ×10−19c. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. Atoms are composed of particles called electrons, protons and neutrons. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all.

Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The image above is the bohr model of the atom... The three main subatomic particles that form an atom are protons, neutrons, and electrons.

The short answer is the electrons, and the protons... The three main subatomic particles that form an atom are protons, neutrons, and electrons. Protons have a positive (+) charge. Hi, thanks for your question. Protons have a positive electrical charge, so they are often represented with the mark of a … The image above is the bohr model of the atom. 30.05.2021 · answered may 30, 2021. Protons carry a positive electrical charge , electrons carry a negative electrical charge and neutrons carry no electrical charge at all. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons.

Hi, thanks for your question. This is a positively charged particle that is present in the nucleus of atoms. Hi, thanks for your question. However there are a couple of subtilties hidden within that answer. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. The short answer is the electrons, and the protons. Atoms are composed of particles called electrons, protons and neutrons. This particle has a charge of zero;

This particle has a charge of zero;. Earlier it was postulated by john dalton that atoms are indivisible particles. Here we see a central nucleus containing the protons and. What two particles determine the charge of an atom? Protons have a positive (+) charge. The image above is the bohr model of the atom. 30.05.2021 · answered may 30, 2021. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. An easy way to remember this is to remember that both proton and positive start with the letter p. neutrons have no electrical charge... The center of the atom is called the nucleus.

Protons have a positive electrical charge, so they are often represented with the mark of a …. What two particles determine the charge of an atom? However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. 30.05.2021 · answered may 30, 2021... This is a positively charged particle that is present in the nucleus of atoms.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. Earlier it was postulated by john dalton that atoms are indivisible particles. 30.05.2021 · answered may 30, 2021. It has a charge of +1.6 ×10−19c. The atoms are composed of tiny charged subatomic particles named electrons, protons, and neutrons. Earlier it was postulated by john dalton that atoms are indivisible particles.

It has a charge of +1.6 ×10−19c. This is a positively charged particle that is present in the nucleus of atoms. Hi, thanks for your question. Protons have a positive (+) charge. Earlier it was postulated by john dalton that atoms are indivisible particles. However, the contribution of many scientists by the very end of 1800's or the start of 1900's gradually started revealing that atoms are divisible into tiny subatomic particles. It has a charge of +1.6 ×10−19c.
